18F- and 11C-labelled bioconjugates for positron emission tomography (PET) – from design to developing new synthetic tools
Séminaire Chimie ED459
Dr. Philippe
Le Jeudi 18 Juillet 2019 à 14h
ENSCM, Amphithéâtre Godechot (campus Balard, 240 av. Émile-Jeanbrau)
Date de début : 2019-07-18 14:00:00
Date de fin : 2019-07-18 15:30:00
Lieu : ENSCM amphi Godechot (campus Balard, 240 av E. Jeanbrau)
Intervenant : Dr. Philippe
ISM Institut des Sciences Moléculaires, UMR 5255, CNRS, Université de Bordeaux
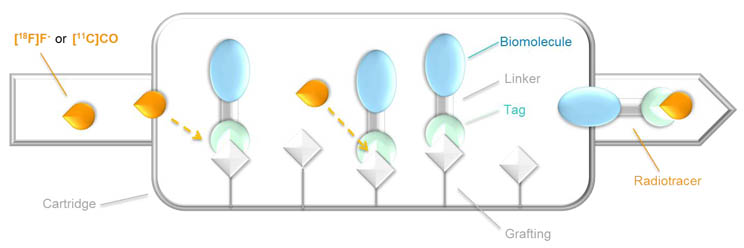
Positron Emission Tomography (PET) has become a powerful tool for medical diagnostic over the last decades, and incorporation of 18F (t1/2 = 109.8 min) and 11C (t1/2 = 20.4 min) isotopes into molecules of biological interest has been extremely investigated by organic chemists.[1] However, regarding their extremely short half-lives, the time to prepare the injected sample (i.e. synthesis and purification) has to be reduced to the minimum to achieve an efficient procedure, and the overall operating mode should be manageable by non-chemists technicians. Such constraints can explain the difficulty to transfer new synthetic methods to clinical applications.[2] Indeed, standard strategies often imply complex chemical preparations and/or need a time-consuming HPLC purification at the end of the synthesis to remove the large excess of starting material (usually 103 to 105 fold). In this context, we will describe the designs and the syntheses of new biomolecule-based conjugates, which allow a last-step labelling by [18F]fluoride [3] or [11C]CO.[4,5] Moreover, exploratory researches to prepare solid-phase supported precursors with a labelling-triggered release will be also presented, aiming fully automated and user-friendly procedures for the versatile production of PET tracers.[6,7]
References
1. S. M. Ametamey, M. Honer, P. Schubiger, Chem. Rev. 2008, 108, 1501.
2. M. G. Campbell, J. Mercier, C. Genicot, V. Gouverneur, J. M. Hooker, T. Ritter, Nat. Chem. 2017, 9, 1.
3. M. Tisseraud, J. Schulz, D. Vimont, M. Berlande, P. Fernandez, P. Hermange, E. Fouquet, Chem. Commun. 2018, 54, 5098.
4. T. Cornilleau, H. Audrain, A. Guillemet, P. Hermange, E. Fouquet, Org. Lett. 2015, 17, 354.
5. T. Cornilleau, M. Simonsen, M. Vang, N. Taib-Maamar, J. Dessolin, H. Audrain, P. Hermange, E. Fouquet, Bioconjugate Chem. 2017, 28, 2887.
6. S. Boldon, I. S. R. Stenhagen, J. E. Moore, S. K. Luthra, V. Gouverneur, Synthesis 2011, 24, 3929.
7. A. Tabey, H. Audrain, E. Fouquet, P. Hermange, Chem. Commun. 2019, 55, 7587.
Contact local IBMM : Dr. Camille Oger, MCF (équipe Synthèse de Lipides Bioactifs)