Fluorinated pseudoprolines as original tools for conformational control of peptide chains
Séminaire Chimie IBMM
Dr. Grégory
Le Mardi 17 Septembre 2019 à 13h15
UM UFR Pharmacie, Amphithéâtre C
Date de début : 2019-09-17 13:15:00
Date de fin : 2019-09-17 14:30:00
Lieu : UM Fac Pharma, amphi C
Intervenant : Dr. Grégory
LCB Laboratoire de Chimie Biologique, EA 4505, Université de Cergy-Pontoise
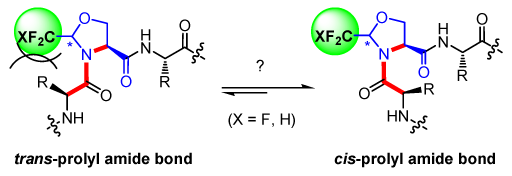
The design of constrained peptides is of prime importance in the development of bioactive compounds and for applications in supramolecular chemistry. Due to its nature, the peptide bond undergoes a spontaneous cis-trans isomerism, and the cis isomers are much more difficult to stabilize than the trans forms. To constrain the amide bond conformation, numerous alkyl-substituted pyrrolidine rings have been introduced as proline surrogates in Xaa-Pro sequences.[1] While they have almost no effect at the Cβ and Cγ positions, Cα and Cδ alkylations lead to a strong preference for the trans and cis isomers, respectively.[2] Furthermore, comprehensive studies on pseudoproline (ΨPro) surrogates have shown that changing the substituent nature, its configuration or the degree of substitution at the Cδ position tuned the cis/trans isomer ratio.[3]
Our group is interested in the development of efficient routes for the preparation of enantiopure fluorinated amino acids [4] and their incorporation into a peptide chain.[5] In particular, we have reported several studies based on CF3-pseudoprolines (CF3-ΨPro) model peptides which established the stereoelectronic effects imparted by the CF3 group at the Cδ position.[6] Very recently, we have also been interested in the synthesis of CF2H-pseudoprolines (CF2H-ΨPro). Here, I will report the preparation of fluorinated pseudoprolines as well as the methodological study developed to optimize their incorporation into peptides. Conformational studies as well as application to collagen model peptides will be shown.
References
1. (a). P. Karoyan, S. Sagan, O. Lequin, J. Quancard, S. Lavielle, G. Chassaing, In: Targets in Heterocyclic Systems-Chemistry and Properties, Eds O. A. Attanasi, D. Spinelli, Royal Society of Chemistry, Cambridge, 2005, pp 216–273 ; (b) Cis-Trans Isomerization in Biochemistry, Ed. C. Dugave, Wiley-VCH, Weinheim, 2006 ; (c) C. Dugave, L. Demange, Chem. Rev. 2003, 103, 2475 ; (d) Y. Che, G. R. Marshall, Biopolymers 2006, 81, 392.
2. (a) M. De Poli, A. Moretto, M. Crisma, C. Peggion, F. Formaggio, B. Kaptein, Q. B. Broxterman, C. Toniolo, Chem. Eur. J. 2009, 15, 8015 ; (b) C. Mothes, C. Caumes, A. Guez, H. Boullet, T. Gendrineau, S. Darses, N. Delsuc, R. Moumné, B. Oswald, O. Lequin, P. Karoyan, Molecules 2013, 18, 2307.
3. P. Dumy, M. Keller, D. E. Ryan, B. Rohwedder, T. Wöhr, M. Mutter, J. Am. Chem. Soc. 1997, 119, 918.
4. (a) G. Chaume, O. Barbeau, P. Lesot, T. Brigaud, J. Org. Chem. 2010, 75, 4135 ; (b) H. Lubin, J. Pytkowicz, G. Chaume, G. Sizun-Thomé, T. Brigaud, J. Org. Chem. 2015, 80, 2700 ; (c) C. Caupène, G. Chaume, L. Ricard, T. Brigaud, Org. Lett. 2009, 11, 209 ; (d) J. Simon, T. T. Nguyen, E. Chelain, N. Lensen, J. Pytkowicz, G. Chaume, T. Brigaud, Tetrahedron: Asymmetry 2011, 22, 309.
5. (a) G. Chaume, N. Lensen, C. Caupène, T. Brigaud, Eur. J. Org. Chem. 2009, 5717 ; (b) G. Chaume, D. Feytens, G. Chassaing, S. Lavielle, T. Brigaud, E. Miclet, New J. Chem. 2013, 37, 1336 ; (c) I. Jlalia, N. Lensen, G. Chaume, E. Dzhambazova, L. Astasidi, R. Hadjiolova, A. Bocheva, T. Brigaud, Eur. J. Med. Chem. 2013, 62, 122 ; (d) J. Simon, J. Pytkowicz, N. Lensen, G. Chaume, T. Brigaud, J. Org. Chem. 2016, 81, 5381 ; (e) G. Chaume, J. Simon, N. Lensen, J. Pytkowicz, T. Brigaud, E. Miclet, J. Org. Chem. 2017, 82, 13602 ; (f) M. Oliver, C. Gadais, J. García-Pindado, M. Teixidó, N. Lensen, G. Chaume, T. Brigaud, RSC Adv. 2018, 8, 14597 ; (g) C. Gadais, K. Van holsbeeck, S. Moors, D. Buyst, K. Fehér, K. Van Hecke, D. Tourwé, T. Brigaud, C. Martin, F. De Proft, J. Pytkowicz, J. C. Martins, G. Chaume, S. Ballet, ChemBioChem, 2019, DOI: 10.1002/cbic.201900294.
6. D. Feytens, G. Chaume, G. Chassaing, S. Lavielle, T. Brigaud, B. J. Byun, Y. K. Kang, E. Miclet, J. Phys. Chem. B 2012, 116, 4069.
Biosketch.
Grégory
Contact local IBMM : Dr. Ludovic