Durability of carbon-supported electrocatalysts in alkaline electrolytes for fuel cell applications
Séminaire Chimie ED459 / atelier LAbEx
Prof. Marian
Le Jeudi 20 Juin 2019 à 14h
ENSCM, Amphithéâtre Godechot (campus Balard, 240 av. Émile-Jeanbrau)
Conférence présentée dans le cadre de l’atelier Labex CheMISyst “Électrochimie à Montpellier pour l’eau, l’énergie et la santé”|programme et détails sur le site du Pôle Balard
Date de début : 2019-06-20 14:00:00
Date de fin : 2019-06-20 15:00:00
Lieu : ENSCM amphi Godechot (campus Balard, 240 av E. Jeanbrau)
Intervenant : Prof. Marian
LEPMI Laboratoire d’Électrochimie et de Physicochimie des Matériaux et des Interfaces, UMR 5279 CNRS, Université Grenoble Alpes
Note aux doctorants ED459 SCB Sciences Chimiques Balard :
Cette conférence étant au programme d’un atelier labellisé par l’ED459, sa prise en compte pour le crédit “Séminaires ED” ne peut pas être cumulée avec la validation de l’atelier en heures de formation doctorale.
Co-authors : Clémence
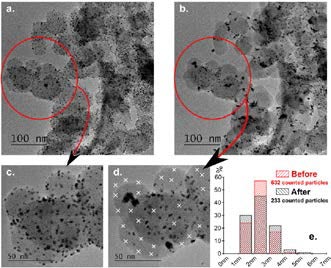
Alkaline fuel cells (AFC) may compete with proton-exchange membrane fuel cells (PEMFC) for stationary or portable applications [1]. The intrinsic stability of many metals and metal oxides at high pH [2] led the scientific community to admit that AFC electrocatalysts should be more stable than PEMFC ones, but it is not the case for carbon-supported Pt and Pd nanoparticles (NPs) aged in liquid alkaline environment: severe electrochemical surface area losses are observed upon mild accelerated stress tests in 0.1 M NaOH [3,4] (Figure). Pronounced detachment of the Pt (and Pd) NPs from the carbon support occurs, but minor degradation of the metal NPs and of the carbon support are observed by identical-location transmission electron microscopy (ILTEM). Additional experiments performed in various alkaline electrolytes (LiOH, NaOH, KOH, CsOH) coupled with in situ Fourier-transform infrared spectroscopy linked this detachment to the formation of solid carbonates at the interface between the Pt (Pd) NPs via carbon support corrosion: in essence, the Pt (Pd) NPs do locally catalyze the carbon support corrosion (firstly carbon surface oxides groups are formed, then evolve into CO2, which leads to the formation of carbonate anions in alkaline environment and finally to metal carbonate precipitation, M2CO3, M = Li, Na, K or Cs) [5].
The loss of Pt NPs is mitigated for solid alkaline electrolytes (anion-exchange membrane), because the counter-cation of the OH- species are immobilized on the polymer backbone and cannot lead to precipitation into M2CO3, which happens in MOH aqueous electrolytes. This minored degradation of the Pt/C NPs in AEM electrolytes does not mean that no degradation processes are at stake, though: Ostwald ripening and Pt redeposition are observed. This means that the nature of the predominant mechanisms of degradation differ in solid versus liquid alkaline environment, which had also been demonstrated for solid versus liquid acidic electrolytes [6,7].
Finally, it will be shown that these degradations are not unavoidable; non-noble electrocataysts (e.g. Ni-based) prove much more resistant to corrosion in alkaline environments [8], and should therefore be more durable in AFC applications.
References
1. E.H. Yu, X. Wang, U. Krewer, L. Li, K. Scott, Direct oxidation alkaline fuel cells: from materials to systems, Energy Environ. Sci., 5 (2012) 5668.
2. M. Pourbaix, Atlas of Electrochemical Equilibria in Aqueous Solutions, in, National Association of Corrosion Engineers, Houston, 1979, pp. 453.
3. A. Zadick, L. Dubau, N. Sergent, G. Berthomé, M. Chatenet, Huge Instability of Pt/C Catalysts in Alkaline Medium, ACS Catal., 5 (2015) 4819.
4. A. Zadick, L. Dubau, U.B. Demirci, M. Chatenet, Effects of Pd Nanoparticle Size and Solution Reducer Strength on Pd/C Electrocatalyst Stability in Alkaline Electrolyte, J. Electrochem. Soc., 163 (2016) F781.
5. C. Lafforgue, F. Maillard, V. Martin, L. Dubau, M. Chatenet, Degradation of Carbon-supported Platinum Group Metal Electrocatalysts in Alkaline Media Studied by in situ Fourier-Transform Infrared Spectroscopy and Identical-Location Transmission Electron Microscopy, ACS Catal., (2019).
6. F.R. Nikkuni, L. Dubau, E.A. Ticianelli, M. Chatenet, Accelerated degradation of Pt3Co/C and Pt/C electrocatalysts studied by identical-location transmission electron microscopy in polymer electrolyte environment, Appl. Catal. B: Environmental, 176-177 (2015) 486.
7. F.R. Nikkuni, B. Vion-Dury, L. Dubau, F. Maillard, E.A. Ticianelli, M. Chatenet, The role of water in the degradation of Pt3Co/C nanoparticles: An Identical Location Transmission Electron Microscopy study in polymer electrolyte environment, Appl. Catal. B: Environmental, 156–157 (2014) 301.
8. A. Zadick, L. Dubau, K. Artyushkova, A. Serov, P. Atanassov, M. Chatenet, Nickel-based electrocatalysts for ammonia borane oxidation: enabling materials for carbon-free-fuel direct liquid alkaline fuel cell technology, Nano Energy, 37 (2017) 248.
Contacts locaux ICGM/IEM : Dr. Steven