Dynamic covalent chemistry with tetrazines – combining the best of both worlds
Séminaire Chimie ED459
Prof. Romen
Le Jeudi 05 Octobre 2023 à 14h
CNRS, Salle Balard-1 (bâtiment Balard RdC, 1919 route de Mende)
[reprogrammation de la conférence annulée le 21 septembre pour raison de santé]
Date de début : 2023-10-05 14:00:00
Date de fin : 2023-10-05 15:30:00
Lieu : CNRS salle Balard-1 (site RdM bât. Balard RdC)
Intervenant : Prof. Romen
CSIC IPNA Instituto de Productos Naturales y Agrobiología, Tenerife, Spain
Keywords: dynamic covalent chemistry, tetrazine, molecular cages, dynamic polymers, drug delivery
The main feature of supramolecular chemistry resides in its dynamic nature: Weak non- covalent interactions can be formed and broken very easily and therefore they are able to reach the thermodynamic equilibrium, allowing some sort of “molecular error checking” by which the system finally organizes in the most preferred way. However, such inherent lability is also the major handicap of supramolecular chemistry, which precludes its use in several applications. Dynamic Covalent Chemistry (DCvC) was developed to combine reversibility and robustness in an optimal way. This kind of chemistry is based on reversible covalent bonds, and therefore extends all the advantages of the supramolecular chemistry to the molecular domain.
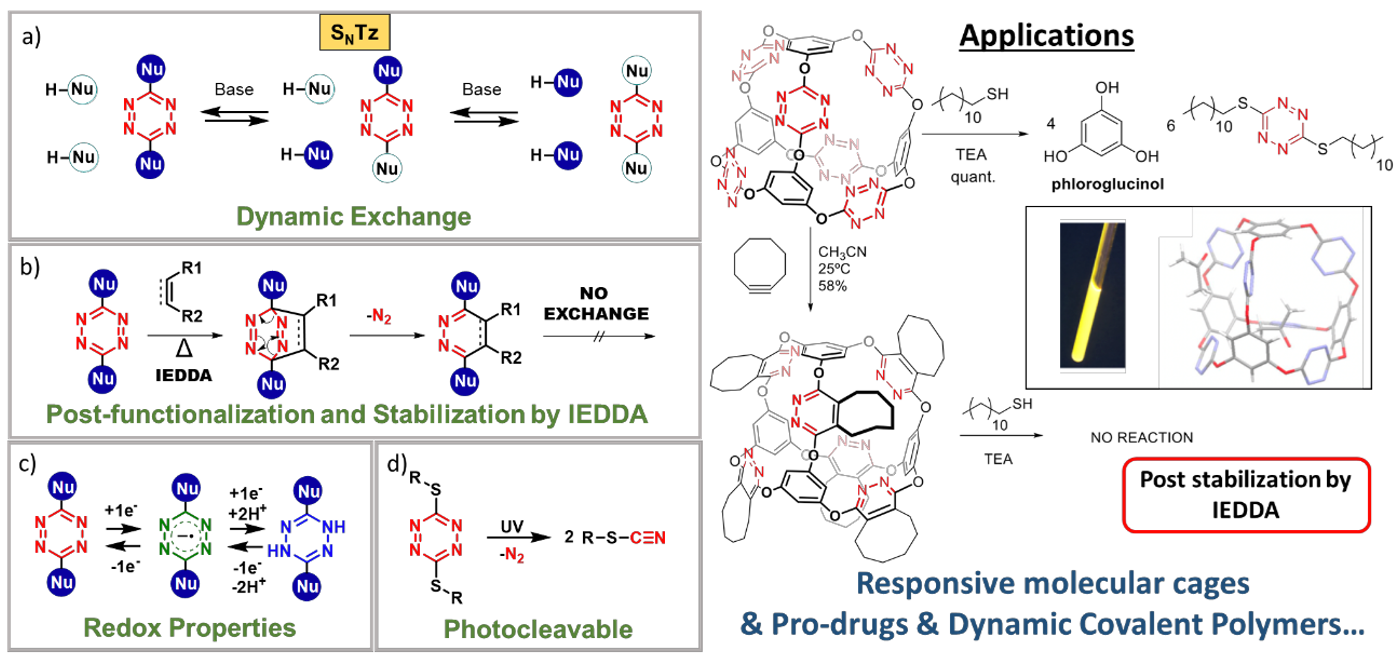
In this regard, our research group recently developed a novel dynamic reaction: The Nucleophilic Aromatic Substitutions of Tetrazines (SNTz), which in addition to its reversible nature, it takes advantage of the versatility of the tetrazine ring.[1] Indeed, this reaction quickly exchanges phenols and alkylthiols in positions 3 and 6, at room temperature (Figure a). This reaction is compatible with aqueous environments, and the compounds obtained by SNTz always display a remarkable redox behavior (Figure c). Thiol derivatives are photodegradable by UV irradiation, yielding the corresponding thiocyanate (Figure d). But probably the most interesting aspect about SNTz is that all the compounds obtained can be functionalized by the inverse electron demand Diels–Alder (IEDDA) reaction, which also locks the exchange (Figure b). Thus, the IEDDA reaction allows for the attachment of molecular fragments and it confers post-stabilization to the chemical structures. Herein we present our latest results in the synthesis of functional supramolecular systems,[1] targeted drug delivery,[2] and dynamic covalent polymers,[3] all of them by SNTz.
Schemes a-d and applications.
References
1. T. Santos, D. S. Rivero, Y. Pérez-Pérez, E. Martín-Encinas, J. Pasán, A. H. Daranas, R. Carrillo, Angew. Chem. Int. Ed. 2021, 60, 18783–18791.
2. M. D. Perretti, Y. Pérez-Pérez, E. Martín-Encinas, C. Alonso-Pérez, J. Scoccia, R. Carrillo, Chem. Commun. 2022, 58, 5518–5521.
3. D. S. Rivero, R. E. Paiva-Feener, T. Santos, E. Martín-Encinas, R. Carrillo, Macromolecules 2021, 54, 10428–10434.
Contact local IBMM : Dr. Sébastien