New tools and uses of dynamic covalent chemistry
Séminaire Chimie ED459
Prof. Max
Le Jeudi 03 Octobre 2019 à 15h
ENSCM, Amphithéâtre Godechot (campus Balard, 240 av. Émile-Jeanbrau)
Date de début : 2019-10-03 15:00:00
Date de fin : 2019-10-03 16:00:00
Lieu : ENSCM amphi Godechot (campus Balard, 240 av E. Jeanbrau)
Intervenant : Prof. Max
Institute of Organic Chemistry and Advanced Materials, University of Ulm, Germany
Dynamic covalent chemistry (DCC) is a powerful tool for probing non-covalent interactions, identifying ligands for medicinally relevant biological targets, and for making use of the feature of “error correction” to achieve the synthesis of interesting molecules and materials.[1]
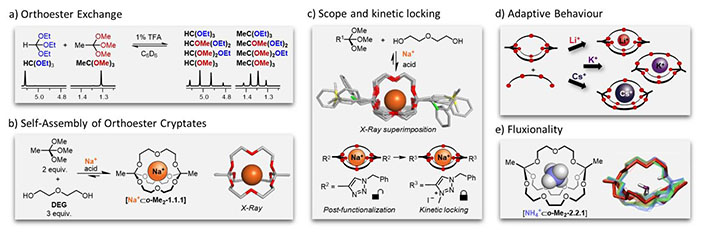
In this talk, I will present our recent work on a previously ignored dynamic covalent reaction: the acid-catalyzed reaction of O,O,O-orthoesters with alcohols (Fig. 1-a),[2a] which we were able to use for the one-pot synthesis of cryptates, in which orthoesters act as tripodal bridgeheads.[2b] Due to their unique structure (Fig. 1-b), these compounds exhibit a range of unusual properties, including tunable, pH-dependent hydrolysis (Fig. 1-c).[2c] Most notably, dynamic orthoester architectures offer an elegant entry to experiments, in which a metal ion selects its preferred host from a dynamic mixture of competing subcomponents (“adaptive host-guest systems”, Fig. 1-d).[2d] Of particular relevance to the area of systems chemistry is our recent discovery that ammonium complexes of orthoester cryptands represent the first example of “fluxional supermolecules”, i.e. these host-guest complexes are inherently dynamic and adaptive (Fig. 1-e).[2 e]
I will close the talk by discussing unpublished work on “new” dynamic covalent reactions and their (potential) uses.
References
1. J.-M. Lehn, “Perspectives in chemistry − aspects of adaptive chemistry and materials”, Angew. Chem. Int. Ed. 2015, 54, 3276.
2. a) R.-C. Brachvogel, M. von Delius, Chem. Sci. 2015, 6, 1399. b) R.-C. Brachvogel, F. Hampel, M. von Delius, Nat. Commun. 2015, 6, 7129. c) H. Löw, E. Mena-Osteritz, M. von Delius, Chem. Sci. 2018, 9, 4785. d) O. Shyshov, R.-C. Brachvogel, T. Bachmann, R. Srikantharajah, D. Segets, F. Hampel, R. Puchta, M. von Delius, Angew. Chem. Int. Ed. 2017, 56, 776. e) X. Wang, O. Shyshov, M. Hanževački, C. M. Jäger, M. von Delius, J. Am. Chem. Soc. 2019, 141, 8868.
Contact local IBMM : Dr. Sébastien