Recent progress in the area of heterocycle synthesis
Séminaire Chimie ED459
Prof. Asunción
Le Jeudi 14 Septembre 2023 à 14h
CNRS, Amphithéâtre Balard (bâtiment Balard RdC, 1919 route de Mende)
[report après annulation de la date initiale 29 juin]
Date de début : 2023-09-14 14:00:00
Date de fin : 2023-09-14 15:30:00
Lieu : CNRS amphi Balard
Intervenant : Prof. Asunción
Department of Organic Chemistry, Faculty of Sciences, University of Valladolid, Spain
Heterocycles are scafolds present in a wide variety of natural products with important medicinal properties. Among them, 6-, 7- and 8-membered heterocycles have attracted special attention due to their occurrence in bioactive compounds of great pharmacological interest. Consequently, a great number of scientifics have reported a variety of synthetic protocols to afford these substrates.
Within the known strategies, Prins cyclization has emerged as a very efficient tool to obtain heterocycles in a very stereoselective manner.[1,2]
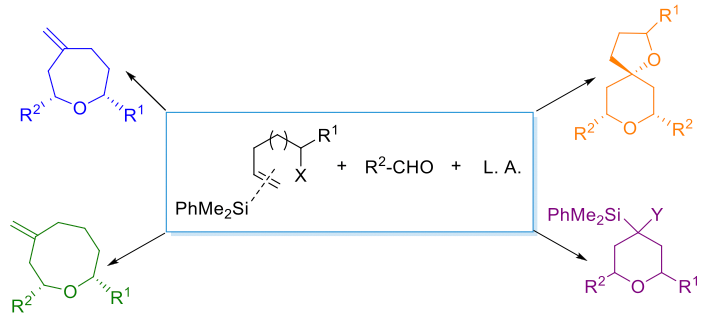
Following our interest in the development of new approaches to the synthesis of heterocyclic structures, promoted by silicon-mediated cyclizations, we now present our recent results in the application of the silyl-Prins cyclization to the synthesis of different sized heterocycles (Scheme 1), including 6-, 7- and 8-membered derivatives.[3–5]
Scheme 1. Towards the synthesis of polysubstituted heterocycles.
References
1. a) C. Olier, M. Kaafarani, S. Gastaldi, M. P. Bertrand, Tetrahedron 2010, 66, 413–445. b) I. Pastor, M. Yus, Curr. Org. Chem. 2007, 11, 925–957. c) X. Han, G. Peh, P. E. Floreancig, Eur. J. Org. Chem. 2013, 1193–1208. d) B. V. S. Reddy, P. N. Nair, A. Antony, C. Lalli, R. Grée, Eur. J. Org. Chem. 2017, 1805–1809.
2. C. Díez-Poza, A. Barbero, Eur. J. Org. Chem. 2017, 4651–4665.
3. a) A. Barbero, A. Diez-Varga, F. J. Pulido, Org. Lett. 2013, 15, 5234–5237. b) A. Diez-Varga, H. Barbero, F. J. Pulido, A. González-Ortega, A. Barbero, Chem. Eur. J. 2014, 20, 14112–14119. c) A. Barbero, A. Diez-Varga, M. Herrero, F. J. Pulido, J. Org. Chem. 2016, 81, 2704–2712. d) A. Barbero, A. Diez-Varga, F. J. Pulido, A. González-Ortega, Org. Lett. 2016, 18, 1972–1975.
4. C. Díez-Poza, A. Barbero, Org. Lett. 2021, 23, 8385–8389.
5. C. Díez-Poza, L. Fernández-Peña, P. González-Andrés, A. Barbero, J. Org. Chem. 2023, in press.
Acknowledgement: we thank the Spanish Ministry of Science and Innovation for financial support.
Contact local IBMM : Dr. Florine