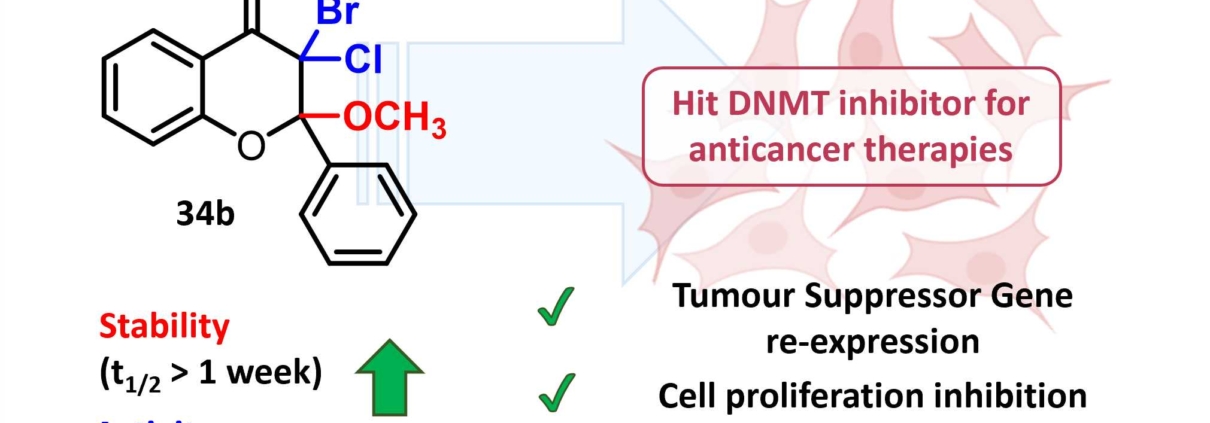
A new potent and stable DNA methyltransferase inhibitor developed by the Glycochemistry & Molecular Recognition team for cancer treatment
The researchers of the Glycochemistry & Molecular Recognition group of the IBMM have just published a paper on the Journal of Medicinal Chemistry, where they show the results of the rational optimisation of their flavanone derivatives as DNA methyltransferase (DNMT) inhibitors. Among the new synthesised compounds, they identified compound 34b, a novel dihalo-methoxyflavanone with high chemical stability and increased activity, which currently represents the most potent DNMT3A inhibitor described so far. Compound 34b increased the levels of tumour suppressor genes and triggered a critical cell cycle arrest in the G1/S transition, notably in p53-depleted colorectal cancer cells. Modulating DNA methylation is a valuable strategy for the epigenetic reprogramming of cancer cells, which makes compound 34b stand out as a novel hit compound for anticancer therapy.
Te see full article : https://doi.org/10.1021/acs.jmedchem.4c02075