The team’s activity is focused on the development of a medical device (Figure 1) for the treatment of solid cancers (particularly breast cancer) and more broadly for studying the cellular migration of tumor cells in vivo and in vitro using molecules, a chemical matrix, and a microfluidic chip.
Our team also works on myeloproliferative syndromes (abnormal proliferation of blood cells in the bone marrow) and is now a reference laboratory for the exploration of myeloproliferative syndromes.
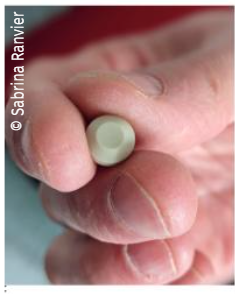
Figure 1: Photograph of our medical device
Our Themes
Capture and Trapping of Metastatic Cells (CAPDCM)
The treatment of solid cancers, particularly breast cancer, relies on conventional therapies such as surgery, radiotherapy, chemotherapy, and more recently, targeted therapy. Despite these advancements, it was estimated that in 2018, 627,000 women worldwide died from breast cancer. Our multidisciplinary team (biology, microfluidics, and chemistry) proposes a novel therapeutic approach targeting operable solid cancers like breast cancer.Our “attract and trap” strategy combines innovations enabling a biocompatible microfluidic chip (less than 1 cm in diameter) to attract and trap cancer cells. At the end of its active period, the implantable medical device (IMD) can be removed. The IMD will be implanted during or immediately after the biopsy. It will cover the preoperative phase (2 to 3 months), perioperative phase (2 days), and postoperative phase (2 months). During these phases, numerous tumor cells may leave the primary tumor, spread to surrounding tissues and other organs, and potentially cause patient relapse.
Development of an analytical platform for molecules and their chemical matrix in the attraction of tumor cells
In partnership with Dr. Benoît Charlot’s team (IES), Dr. Laurent Henry is developing a microfluidic chip that allows the analysis of the attracting power of up to seven chemokines, alone or in combination, in the absence or presence of a chemical matrix on human tumor cells.
The ultimate goal is to study new cytokines or molecules with attracting power to include them in our medical device developed in theme 1 (above).
Functional characterization of mutations in genes associated with myeloproliferative neoplasms (National reference laboratory for the exploration of myeloproliferative syndromes)
This research project is a clinical project in continuity with the biological activity of the Clinical Cytology and Cytogenetics laboratory of the Nîmes University Hospital. It is part of the laboratory labeled as a national reference laboratory in the exploration of myeloproliferative syndromes. Since 2019, we systematically analyze mutation research requests in the context of Myeloproliferative Syndromes (MPS) using a panel targeting major genes, JAK2, cMPL, CALR, and a “prognostic” gene ASXL1, on all exons. This has allowed us to describe numerous variants, most of which are not described in databases or literature. Two genes concentrate a great diversity of novel variants, cMPL and ASXL1. Given the phenotypic effect of cMPL (thrombopoietin receptor, TPO) on the pathologies of interest, we decided to focus on studying these variants. The aim of this project is to establish a capacity for analyzing variants generated by the laboratory’s “routine” activity.









