The team ‘Analytical Sciences of Biomolecules’ of IBMM (F12), led by C. Enjalbal, is belonging to the Department “Acides Aminés, Peptides & Protéines” (DAPP).
The team is currently gathering 11 permanent staff (6 EC, 1C, 3 BIATSS and 1 ITA) whose expertise are all linked to analytical sciences.
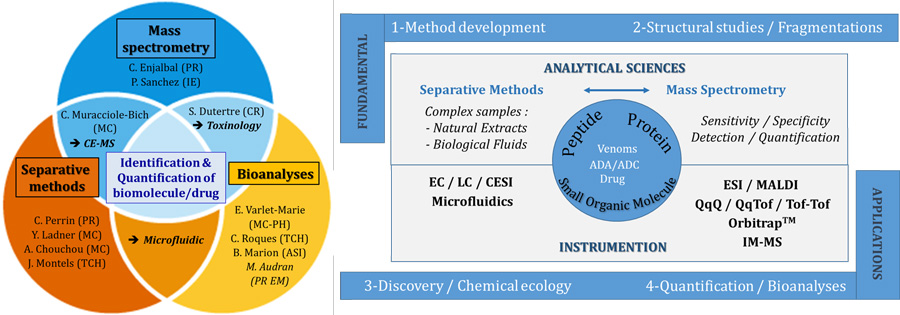
Team F12 is benefiting from complementary expertise to tackle analytical issues in chemical ecology and pharmacy. Many ongoing fundamental and applicative researches dealing with mass spectrometry, liquid chromatography, capillary electrophoresis and microfluidic are conducted for the detection and quantification in complex samples of peptides, proteins, toxins, antibodies, as well as small organic drugs, in the context of pharmaceutical and therapeutic issues (fragmentation studies, optimization/validation of detection and quantification methods).
To reflect the variety of scientific knowledge mastered by the workforce, the team is organized in three groups: Mass spectrometry, Separative Methods and Omics strategies.
Regarding pieces of equipment, separative instruments (1 HPLC, 5 CE, 1 CESI) available within the team can be hyphenated to many mass spectrometers located at the LMP platform. These instruments are fitted with an ambient ionization source (ESI/APCI) and presenting different mass analyzer configurations (ion trap (IT) for iterative dissociations (ESI-MSn), triple quadrupole (QqQ) for bioanalyses (ESI-MS/MS), time of flight (QTof) coupled to ion mobility for high resolution multimodal analyses (ESI-IM-MS/MS)).
Team F12 is benefiting from complementary expertise allowing fundamental and applicative research related to pharmaceutical and therapeutic issues involving analyses of peptides and proteins as targeted biomolecules, and to a less extent small organics (drugs, doping substances) as bioactive compounds. Thus, most of the research topics of F12 are related to method developments for qualitative and quantitative analyses in complex biological media (plasma, urine, cell lysates, natural extracts such as venoms).

Our Themes
Questioning chirality in therapeutic peptides
Questioning chirality in therapeutic peptides: Effect of Asp, His and Gly? – GLYMS (AAPG 2023 ANR – 2023 2026). Investigator
The GLYMS project aims to provide analytical tools to tackle a delicate issue encountered in pharmacology dealing with the chirality of the constitutive amino acids of therapeutic peptides that represents a subtle structural feature very difficult to probe, especially at trace level (0.1%). The GLYMS project is related to method developments for qualitative and quantitative analyses of peptides present in highly complex biological media.
Gathering required expertise in separative technologies, mass spectrometry, and possessing the appropriate samples (home-made peptide library) or being able to prepare any required sequences, the GLYMS PRME project aims to focus on the ultimate highly subtle distinction of diastereoisomeric sequences (same elemental compositions and primary structures) to monitor the chiral integrity of therapeutic peptides. Two amino acids that are known to be prone to epimerization (aspartic acid and histidine) will be selected for model studies. Questioning optical activity on peptide characterization implies that glycine, the sole non chiral residue, must be also included to allow a complete overview of all critical features.
Enzymatic Remodeling of the Extracellular Matrix
Enzymatic Remodeling of the Extracellular Matrix: Experiments in Synthetic Crowded Mimics to Build Comprehensive Kinetic Models – X-CROWD (AAPG 2021 ANR – 2021 2025). Partner
La matrice extracellulaire (MEC) est essentielle pour le fonctionnement des organismes multicellulaires. Des maladies majeures, comme le cancer, sont associées à l’altération de son remodelage dynamique régulé par voie enzymatique. Cependant, celui-ci est encore peu compris au niveau théorique dans des conditions réalistes. Dans ce projet, nous étudierons comment la cinétique de certaines enzymes de la MEC est influencée par des milieux artificiels contrôlés incluant des composants clés de la MEC, qui sont aussi des substrats pour les enzymes, comme le collagène et l’acide hyaluronique. En partant de travaux collaboratifs préliminaires, nous réaliserons des analyses par spectroscopie UV/Vis, électrophorèse capillaire couplée à la mobilité ionique et à la spectrométrie de masse et RMN PFG sur des systèmes de complexité croissante. À terme, ce projet produira d’innovants modèles cinétiques de dégradation/remodelage enzymatique de composants clés de la MEC en conditions proches du réel.
Development of miniaturized analytical tools for Therapeutic Drug Monitoring
Therapeutic Drug Monitoring (TDM) is the clinical practice of monitoring active substance concentrations at defined intervals to maintain a constant concentration in a patient’s bloodstream, with a view to optimizing individual dosage regimens. This approach is based on the existence of a relationship between active substance concentration and biological effect. This practice is particularly appropriate when administering drugs with a narrow therapeutic index, i.e. drugs for which the difference between the active concentration (responsible for therapeutic efficacy) and the toxic concentration (responsible for the appearance of undesirable effects) is small. TDM allows individualization of drug dosage by maintaining plasma or blood drug concentrations within a targeted therapeutic window. Implementing TDM reduces the number of adverse effects for patients, which in turn reduces the cost of care for institutions.
TDM is a major public health issue, particularly for cancer treatment. Today, these assays often require the use of heavy, expensive analytical equipment (such as liquid chromatography coupled with UV spectroscopy or tandem mass spectrometry (LC-MS/MS)), and the time required to deliver results is long (several days between sample collection and result), forcing patients to make regular visits to overcrowded health services. All these aspects make it difficult to set up a TDM scan, putting patients at risk.
The aim of the current project is to develop a new analytical tool that is simple, fast, inexpensive and autonomous, enabling direct measurement by the patient of blood concentrations of various therapeutic molecules, with a view to individualizing dosage adjustments in real time. The aim of the thesis will be to develop miniaturized analytical systems (based on µfluidic technology) dedicated to the analysis of plasma concentrations of drugs requiring personalized TDM. The systems developed should meet one of the main challenges of TDM, namely fully integrated operation, from sample preparation to plasma concentration determination.
A microfluidic device based on laser technology and using an innovative resin with specific physico-chemical properties will be developed. The miniaturization of sample preparation (protein precipitation, desalting, extraction) will be achieved for the first time (never published but feasibility tests carried out in-house) by combining “Droplet microfluidics” technology and the SALLE (Salting-out Assisted Liquid-Liquid Extraction) approach.
Analytical method development for a therapeutic VHH-oligonucleotide conjugate
Analytical method development for a therapeutic VHH-oligonucleotide conjugate: characterization, stability and quantification in biological media. Industrial partnership
Vect-Horus is a private biotechnology company that designs and develops vectors to facilitate the targeted delivery of therapeutic molecules and imaging agents. Specifically, Vect-Horus has developed and optimized a family of VHH-type vectors targeting the transferrin receptor (TfR). In the context of its R&D activities, Vect-Horus conjugates these VHHs with therapeutic oligonucleotides to enhance their potential by increasing their delivery to TfR-overexpressing tissues. Various conjugate designs have been tested. Among these, a heterodimeric format using the Fc fragment as a platform has been identified. The final design is based on an Fc region of the “knobs-into-holes” type, fused on one hand to a VHH targeting the TfR and on the other hand to a peptide tag used for enzymatic conjugation to small interfering RNAs (siRNA). These VHH-hFc-siRNA conjugates have demonstrated their potentiel to silence the target gene in the tissues of interest. Vect-Horus now aims to develop analytical tools for the characterization and quantification of these conjugates in order to study their chemical and biological stability, understand their degradation profile, and be able to quantify the intact molecule and its metabolites in various biological environments such as tritosomes or tissues of interest. This thesis project will consist of several steps. In the initial phase, the existing literature on available analytical techniques for VHH conjugates and oligonucleotides, their in vitro and in vivo stability, and the applied methods will be researched. The objective is to build on existing techniques to develop those most suitable for our original conjugates.
In the second phase, a stable and robust method will be developed for the characterization of the VHH-hFc-siRNA conjugate in its storage buffer. This will provide the impurity profile of the conjugate according to the internal manufacturing process forming the basis for subsequent steps. Next, the study will focus on the degradation of the VHH-hFc-siRNA conjugate under different conditions (storage conditions and forced conditions). The monitoring of the potential degradation of the conjugate will be conducted for each condition over 18 months, along with potential degradation products.
Using the information obtained from the stability study in a buffered environment, the monitoring of stability in a biological environment (plasma/blood/urine) artificially spiked with the conjugate will be conducted. If necessary, the method will be optimized to enhance sensitivity toward the compound. Sample preparation, detection, and quantification using an internal standard will lead to a validated method.
Finally, the validated method will be used in a pharmacokinetic study of the VHH-hFc-siRNA conjugate in mice and further develop a quantification method in tissues to assess its in vivo biodistribution.
These data will be crucial for anticipating the development of the VHH-hFc-siRNA conjugate by Vect-Horus. Furthermore, this will open up the possibility of studying the biodistribution of this type of conjugate, offering the potential for using this methodology to optimize VHH-hFc-siRNA conjugates in different applications.


 Christine ENJALBAL
Christine ENJALBAL










