Home > Group members [fr] > Robert Pascal
DSBC – Robert Pascal personal web page
From May 1st, 2019, Robert Pascal has been appointed Emeritus Sr Scientist in the ASTRO group of PIIM laboratory in Marseille. This page is no longer updated.

Robert Pascal
CNRS Senior Scientist Emeritus (DR2 ém. CNRS)
Doctorat d’État, University of Montpellier, France (1980)
E-mail: robert.pascal![]() univ-amu.fr
univ-amu.fr
ORCiD : 0000-0001-9579-2503
ResearcherID : O-1559-2013
Research Interests:
Current projects:
- PeptiSystems (funded by the ANR)
- Coevolution of RNA and peptides – connecting the activation processes (funded by the Simons Foundation through a post-doctoral fellowship to Dr. Ziwei Liu)
Articles unavailable online
- Selected important references published before 1995 in the Nouveau Journal de Chimie or in the Bulletin de la Société Chimique de France, currently unavailable online, will be provided as PDF fac-simile upon request.
- See complete list of publications on ResearcherID.
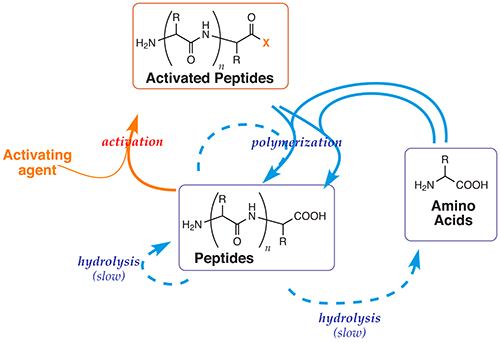
Complexity in chemical systems – Origins and emergence of life
Using theoretical and experimental approaches, we investigate the paths by which chemical systems can lead to an organized behaviour. Our research is aimed at a better understanding of both the principles that allow life to self-organize and of the chemical processes involved. Rather than relying on the occurrence of a highly improbable contingent event that would violate the 2nd Law of thermodynamics, we endorse the idea of a driving force capable of leading a process of self-organisation that is not fundamentally different from biological evolution [A. Pross, What is Life, Oxford University Press, 2012]. Our ‘Systems Chemistry’ approach to origin of life studies is based on the study of reaction networks involving multiple connections rather than that of the synthesis and characterization of individual molecular entities. Our research is aimed at processes occurring in far-from-equilibrium states that lead to reproduction. We address several scientific questions with respect to the origin of life:
- How energy can be brought about to a dynamic chemical system?
- What could be the role of amino acids and peptides in the origin of life process?
- What kind of interaction can be established between the prebiotic and early biochemistry of peptides and nucleotides?
- How did the chirality of amino acids emerge?
Selected References
(DOI links marked with an icon , denote an open-access article)
R. Pascal, I. A. Chen,
From soup to peptides.
[invited News & Views commentary]
Nat. Chem. 2019, 11, 763–764.
DOI: 10.1038/s41557-019-0318-6
R. Pascal,
A possible prebiotic basis for metabolism.
[invited News & Views commentary]
Nature 2019, 569, 47–49.
DOI: 10.1038/d41586-019-01322-3
A. D. Pressman, Z. Liu, E. Janzen, C. Blanco, U. F. Muller, G. F. Joyce, R. Pascal, I. A. Chen,
Mapping a systematic ribozyme fitness landscape reveals a frustrated evolutionary network for self-aminoacylating RNA.
J. Am. Chem. Soc. 2019, 141(15), 6213–6223.
DOI: 10.1021/jacs.8b13298
Z. Liu, G. Ajram, J.-C. Rossi, R. Pascal,
The chemical likelihood of ribonucleotide-α-amino acid copolymers as players for early stages of evolution.
J. Mol. Evol. 2019, 87(2–3), 83–92.
DOI: 10.1007/s00239-019-9887-7
Z. Liu, J.-C. Rossi, R. Pascal,
How prebiotic chemistry and early life chose phosphate.
Life 2019, 9(1), 26.
DOI: 10.3390/life9010026
N. Abou Mrad, G. Ajram, J.-C. Rossi, L. Boiteau, F. Duvernay, R. Pascal, G. Danger,
The prebiotic C-terminal elongation of peptides can be initiated by N-carbamoyl amino acids.
Chem. Eur. J. 2017, 23(31), 7418–7421.
DOI: 10.1002/chem.201700702
A. Pross, R. Pascal,
How and why kinetics, thermodynamics, and chemistry induce the logic of biological evolution.
Beilstein J. Org. Chem. 2017, 13, 665–674.
DOI: 10.3762/bjoc.13.66
Z. Liu, L. Rigger, J.-C. Rossi, J. D. Sutherland, R. Pascal,
Mixed anhydride intermediates in the reaction of 5(4H)-oxazolones with phosphate esters and nucleotides.
Chem. Eur. J. 2016, 22(42), 14940–14949.
DOI: 10.1002/chem.201602697
□ 2e de couverture (inside cover).
Chem. Eur. J. 2016, 22(42), 14758.
DOI: 10.1002/chem.201603523
S. Murillo Sánchez, D. Beaufils, J. M. González Mañas, R. Pascal, K. Ruiz-Mirazo,
Fatty acids’ double role in the prebiotic formation of a hydrophobic dipeptide.
Chemical Science 2016, 7(5), 3406–3413.
DOI: 10.1039/c5sc04796j
D. Beaufils, S. Jepaul, Z. Liu, L. Boiteau, R. Pascal,
The activation of free dipeptides promoted by strong activating agents in water does not yield diketopiperazines.
Orig. Life Evol. Biosph. 2016, 46(1), 19–30.
DOI: 10.1007/s11084-015-9455-0
R. Pascal, A. Pross,
Stability and its manifestation in the chemical and biological worlds.
Chem. Commun. 2015, 51(90), 16160–16165.
DOI: 10.1039/C5CC06260H
R. Pascal,
Kinetic barriers and self-organisation of life.
Isr. J. Chem. 2015, 55, 865–874.
DOI: 10.1002/ijch.201400193
Z. Liu, D. Beaufils, J.-C. Rossi, R. Pascal,
Evolutionary importance of the intramolecular pathways of hydrolysis of phosphate ester mixed anhydrides with amino acids and peptides.
Sci. Rep. 2014, 4, 7440.
DOI: 10.1038/srep07440
D. Beaufils, G. Danger, L. Boiteau, J.-C. Rossi, R. Pascal,
Diastereoselectivity in 5(4H)-oxazolone-mediated prebiotically relevant peptide coupling.
Chem. Commun. 2014, 50(23), 3100–3102.
DOI: 10.1039/c3cc49580a
A. Pross, R. Pascal,
The nature and mathematical basis for material stability in the chemical and biological worlds.
J. Syst. Chem. 2014, 5, 3.
DOI: 10.1186/1759-2208-5-3
R. Pascal, A. Pross, J. D. Sutherland,
Towards an evolutionary theory of the origin of life based on kinetics and thermodynamics.
Open Biol. 2013, 3, 130156.
DOI: 10.1098/rsob.130156
A. Pross, R. Pascal,
The origin of life: what we know, what we can know, what we will never know.
Open Biol. 2013, 3, 120190.
DOI: 10.1098/rsob.120190
G. Danger, A. Michaut, M. Bucchi, L. Boiteau, J. Canal, R. Plasson, R. Pascal,
5(4H)-Oxazolones as intermediates in the carbodiimide- and cyanamide- promoted peptide activations in aqueous solution.
Angew. Chem. Int. Ed. 2013, 52, 611–614.
DOI: 10.1002/anie.201207730
R. Pascal,
Life, metabolism and energy.
In: I. W. M. Smith, C. Cockell, S. Leach (Eds), Astrochemistry and Astrobiology: Physical Chemistry in Action, Springer, Berlin Heidelberg, 2012, pp. 243–269. | ISBN 978-3-642-31729-3. DOI: 10.1007/978-3-642-31730-9_8
R. Pascal,
Suitable energetic conditions for dynamic chemical complexity and the living state.
J. Syst. Chem. 2012, 3, 3.
DOI: 10.1186/1759-2208-3-3
G. Danger, R. Plasson, R. Pascal,
Pathways for the formation and evolution of peptides in prebiotic environments.
Chem. Soc. Rev. 2012, 41, 5416–5429.
DOI: 10.1039/C2CS35064E
R. Pascal, L.Boiteau,
Energy flows, metabolism, and translation.
Philos. Trans. R. Soc. London B, Biol. Sci. 2011, 366, 2949-2958.
DOI: 10.1098/rstb.2011.0135
Organic reactivity / Catalysis / Induced intramolecularity
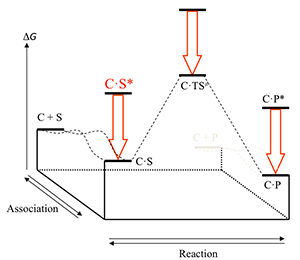
From a more general point of view we are also interested in issues in relationship with physical organic chemistry and especially that of catalysis. An important part of the activity of enzymes is related to the use of binding energy of parts of the substrates that are distant from the reaction centre to bring about catalysis [W.P. Jencks, Adv. Enzymol. Relat. Areas Mol. Biol. 1975, 43, 219–410]. This contribution is required to exceed a kinetic limitation in the formation of reactive encounter complexes. This is not an attribute specific to enzymes and non-covalent interactions and we have shown its efficiency in organic catalysis through the formation of covalent adducts. Induced intramolecularity is therefore an important process to bring about catalysis in chemistry and it has demonstrated efficiency in stereoselective reactions [K.L. Tan, ACS Catal. 2011, 1, 877–886]. It is involved, although this is generally overlooked, in general acid-base catalysis, when proton transfers take place through a hydrogen bond, explaining why intramolecular general acid-base catalysis usually gives rise to low effective molarities.
Selected References
R. Pascal,
Catalysis through induced intramolecularity: what can be learned by mimicking enzymes with carbonyl compounds that covalently bind substrates?
Eur. J. Org. Chem. 2003, 1813–1824. DOI: 10.1002/ejoc.200200530
R. Pascal,
Do enzymes bind their substrates in the ground state because of a physico-chemical requirement?
Bioorg. Chem. 2003, 31, 485-493. DOI: 10.1016/S0045-2068(03)00102-0
R. Pascal,
Induced intramolecularity in the reference reaction can be responsible for the low effective molarities of intramolecular general acid-base catalysis.
J. Phys. Org. Chem. 2002, 15, 566–569. DOI: 10.1002/poc.512
Peptide Chemistry
Our organic reactivity studies have led to processes in which selected reactions of solid- and solution-phase syntheses are performed in aqueous solution. A similar approach has led to the introduction of dendritic lysine polymers.
Selected References
B. Maret, T. Régnier, L. Garrelly, J.-C. Rossi, L. Vial, R. Pascal,
Reduction with tris(2-carboxyethyl)phosphine (TCEP) enables the use of S-sulphonate protecting group for thiol-mediated bioconjugation.
RSC Adv. 2014, 4, 7725–7728.
DOI: 10.1039/c3ra47407k
H. Collet, E. Souaid, H. Cottet, A. Deratani, L. Boiteau, G. Dessalces, J.-C. Rossi, A. Commeyras, R. Pascal,
An expeditious, multi-gram scale synthesis of lysine dendrigraft (DGL) polymers by aqueous N-carboxyanhydride polycondensation.
Chem. Eur. J. 2010, 16, 2309–2316. DOI: 10.1002/chem.200901734
R. Pascal, R. Sola,
Preservation of the Fmoc protective group under alkaline conditions by using CaCl2. Applications in peptide synthesis.
Tetrahedron Lett. 1998, 39, 5031–5034.
DOI: 10.1016/S0040-4039(98)00994-0
Books

Young Sun, Early Earth and the Origins of Life.
Muriel Gargaud, Hervé Martin, Purification Lopez-Garcia, Thierry Montmerle, Robert Pascal,
Springer, Dordrecht, 2013, 300 pp. |
ISBN 978-3-642-22551-2 |
publisher details
Adapted from french: Le Soleil, la Terre… la vie – la quête des origines, Belin, 2009. Details…
Outreach and media
Online videos of lectures (in french), outreach contributions and media appearences, are detailed in the french version.
Links
- Société Française d’Exobiologie (French Astrobiology Society)
- ISSOL – The International Astrobiology Society
- EU COST Chemistry Action CM1304 Emergence and Evolution of Complex Chemical Systems
- EU COST TransDomain Action TD1308 Life-Origins
- Simons Collaboration on the Origins of Life (Simons Foundation, USA)
Updated: April 2019